Abstract
INTRODUCTION: CTCLs such as primary cutaneous anaplastic large-cell lymphomas (pcALCLs) uniformly express CD30, whereas mycosis fungoides (MF) heterogeneously express CD30. In ALCANZA (Horwitz 2021), patients with CD30-positive pcALCL or MF randomized to brentuximab vedotin (BV) vs physician's choice of methotrexate or bexarotene had a significantly higher objective response rate (ORR) lasting ≥4 months (ORR4; 54.7% vs 12.5%; P<0.001) and longer median progression-free survival (PFS; 16.7 vs 3.5 months; P<0.001) and time to next treatment (TTNT; 14.2 vs 5.6 months; P<0.001) at a median follow-up of 45.9 months. This study describes real-world patient characteristics, treatment patterns, clinical outcomes, and healthcare resource use (HRU) in CTCL patients treated with BV or other standard therapies (OST) after ≥1 systemic therapy.
METHODS: A retrospective, longitudinal physician chart review was conducted from Oct 2021-Jan 2022 (approved by WCG IRB). Physicians were oncologists/hematologists with access to complete medical records for ≥1 eligible patient. Eligible adult patients had pcALCL or MF, previously received ≥1 systemic therapy, and were treated with BV or OST from Nov 2017-Mar 2021. The index date was the initiation of second-line (2L) CTCL systemic therapy. The observation period was from index to the earliest of death, loss to follow-up, or chart abstraction. Treatment patterns were assessed from CTCL diagnosis to end of observation period; clinical outcomes for 2L systemic therapy and HRU were assessed during the observation period. Treatment response was recorded via the Global Response Score (Olsen 2011). TTNT was from index to the earlier of initiation of a new line of therapy or death. Real-world PFS (rwPFS) was from index to earliest of progression, treatment discontinuation due to progression, or death. Overall survival (OS) was from index to death. Median times-to-event were computed using Kaplan-Meier (KM) analysis. Since median times-to-event were not reached in both cohorts for multiple outcomes, restricted mean survival time (RMST), a measure of the average event-free survival time during a specific period estimated by the area under the KM curve, at 1 and 2 years was also estimated.
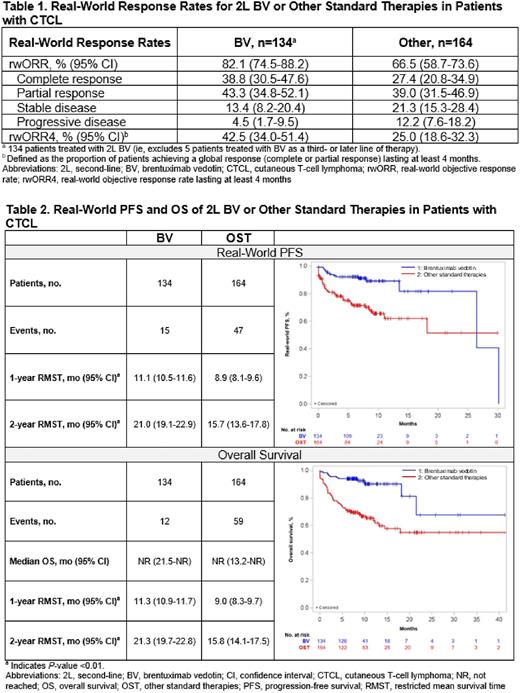
RESULTS: Physicians (n=125; 68.0% community, 75.2% practiced >10 years) abstracted medical charts for 303 patients (BV/OST: n=139/164; male, 66.9%/67.7%; MF, 56.8%/65.9%; ECOG PS 0-1, 86.3%/67.1%). The median observation period for BV and OST was 8.1 (interquartile range: 7.1-10.8) and 8.8 (4.8-11.5) months. Most patients in the BV cohort (96.4%) received BV as 2L systemic therapy. In the OST cohort, the most common 2L therapies were methotrexate (11.6%), mogamulizumab (9.1%), and bendamustine (9.1%) monotherapies. The median (95% CI) duration of 2L therapy for BV and OST was 8.4 (7.1-15.4) and 5.2 (3.9-7.3) months. The rwORR for BV and OST was 82.1% and 66.5%; rwORR4 was 42.5% and 25.0% (Table 1). Median (95% CI) TTNT was not reached (NR; 17.5-NR) for BV and was 13.2 (9.2-NR) months for OST; RMST for TTNT was higher for BV vs OST (1-year: 11.2 [10.8-11.7] vs 8.1 [7.4-8.9] months, P<0.01; 2-years: 20.5 [18.6-22.4] vs 13.7 [11.9-15.5] months, P<0.01). RMST (95% CI) for rwPFS for BV and OST at 1 year was 11.1 (10.5-11.6) vs 8.9 (8.1-9.6) months (P<0.01) and at 2 years was 21.0 (19.1-22.9) vs 15.7 (13.6-17.8) months (P<0.01) (Table 2). Median OS was NR in both cohorts; for BV and OST, 9% and 36% of patients died. RMST (95% CI) for OS for BV and OST at 1 year was 11.3 (10.9-11.7) vs 9.0 (8.3-9.7) months (P<0.01) and at 2 years was 21.3 (19.7-22.8) vs 15.8 (14.1-17.5) months (P<0.01). During the observation period, 30.2% and 39.0% of the BV and OST cohorts had an emergency department visit, and 29.5% and 36.6% had a hospitalization; median (95%CI) hospital length of stay for those with ≥1 hospitalization was 13.7 (CI: 12.6-14.9) and 21.1 (19.9-22.4) days.
CONCLUSION: Real-world outcomes with BV in patients with CTCL who received ≥1 prior systemic therapy were consistent with results from ALCANZA, demonstrating favorable clinical outcomes with BV.
Disclosures
Barta:Affimed: Consultancy; Janssen: Other: Independent Data Monitoring Committee member; Acrotech: Honoraria; Kyowa Kirin: Consultancy, Honoraria; Seagen: Honoraria; Daiichi Sankyo: Consultancy. Liu:Seagen, Inc: Current Employment, Current equity holder in publicly-traded company. DerSarkissian:Analysis Group: Current Employment; AstraZeneca: Research Funding; Takeda: Other: I am an employee of Analysis Group, Inc, which received research funding from Takeda for this study. Chang:Seagen, Inc: Research Funding. Ye:Seagen, Inc: Research Funding. Duh:Pharmacyclics: Research Funding; Apellis Pharmaceuticals: Research Funding; Blueprint Medicine: Research Funding; AstraZeneca: Research Funding; Takeda Oncology: Research Funding; GSK: Research Funding; Merck: Research Funding; Shire: Research Funding; Novartis: Research Funding. Surinach:Seagen, Inc: Consultancy. Fanale:Seagen, Inc: Current Employment, Current equity holder in publicly-traded company. Yu:Seagen, Inc: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal